CryoEM Current Practices: Previous webinars

Some tips & tricks of note:
Sample preparation: tRNA scaffolds and Fab chaperones for RNA structure determination. Grid pre-treatments to increase particle density in grid holes. chameleon grid preparation.
Data processing: Multi-class ab initio to sort heterogeneity. Iterative workflows to obtain a best particle stack.
Complementary techniques: X-ray crystallography to obtain structures of sub-domains. SAXS analysis to verify folding.

Some tips & tricks of note:
Sample preparation: Negative stain EM to characterize oligomeric states.
Data collection: Minimizing dose during data collection to resolve flexible regions of a protein. Use of screening to ensure high quality data.
Data processing: Use of symmetry in 2D classification to understand binding stoichiometry.
Complementary techniques: Comparison to X-ray crystal structures to understand biophysical behavior and answer biological questions.
Other: Best use of local resources for impactful use of national center resources.
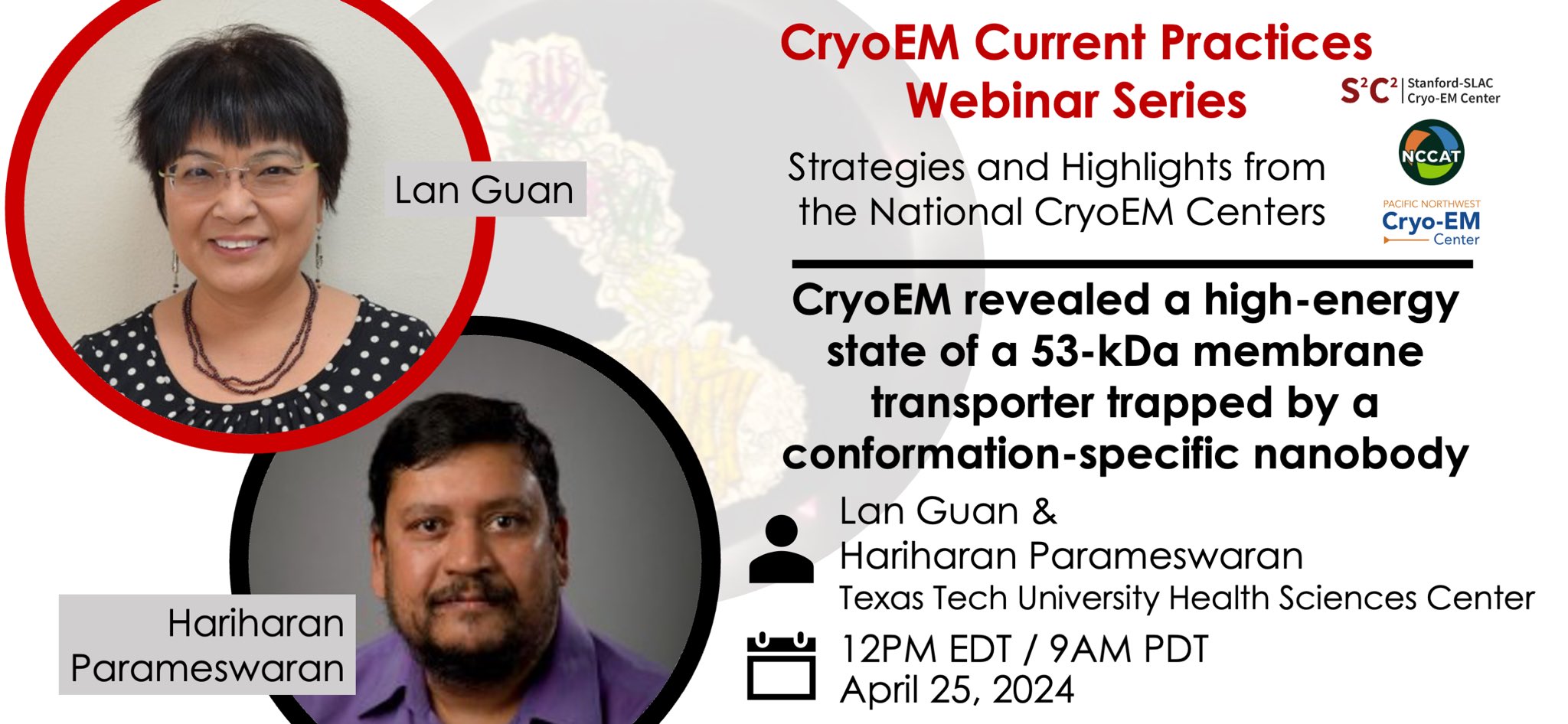
Some tips & tricks of note:
Sample preparation: NabFab strategy for particle picking and alignment of small membrane proteins. Nanodiscs for integral membrane proteins.
Data collection: Tilted data collection.
Data processing: Q-score to evaluate resolution. Processing a sample with heterogeneous conformations to find high-energy states.
Complementary techniques: HDX-MS & MD to characterize Nanobody (Nb) binding & conformational states. Nb characterization by two-hybrid assay. ITC to characterize NabFab and ligand binding to Nb bound transporter.
Some tips & tricks of note:
Sample preparation: Mixed expression systems to reconstitute complexes in vitro. Use of detergents to solve aggregation during plunge freezing.
Data processing: Topaz picking to obtain clean particle stacks. Focused refinement and composite maps for model building and refinement.
Complementary techniques: NMR to assign sequence registry in models when density is weak.
Some tips & tricks of note:
Data processing: Symmetry expansion and focused classification. Use of consensus maps for model building. Visualizing small ligand binding.
Complementary techniques: AI protein design, selection of target binding DARPins.

Some tips & tricks of note:
Sample preparation: Tag locations on heterologously expressed protein constructs. Time resolved cryo-EM from on-grid enzymatic reactions.
Data processing: Handling heterogeneous oligimerization. 3D Variability Analysis (3DVA). Ensemble generation from 3DVA. Assigning small ligand densities.
Complementary techniques: Crosslinking to validate oligomerization interfaces. Enzyme kinetics. Molecular Dynamics Ensemble Refinement. Using cryoEM results to drive development of Undergraduate CURE lab courses.

Some tips & tricks of note:
Sample preparation: Tips for dealing with low sample concentration, samples that stick to carbon, recognizing and troubleshooting strange types of ice.
Data collection: Using information from different magnifications to characterize grids and optimize data collection.
Data processing: How to do gain correction when you don’t have gain references. Helical processing challenges.

Tips & tricks of note:
Sample preparation: membrane protein purification using GDN & nano-disks, expression of leaky ion channels.
Data processing: multi-software workflows, masking and local refinement, symmetry expansion, variability analysis.
Complimentary techniques: patch clamping, single amino acid substitution to verify binding sites.
Tips & tricks of note (coming soon):
Sample preparation:
Data processing:
Complimentary techniques:
Tips & tricks of note (coming soon):
Sample preparation:
Data processing:
Complimentary techniques:
Tips & tricks of note:
Sample preparation: Continuous thin carbon substrates to reduce aggregation.
Data processing: Local refinement to improve map quality in regions of interest.
Complimentary techniques:

Tips & tricks of note:

Tips & tricks of note:
Sample preparation: Anaerobic Grid preparation.
Data processing: “In silico purification” of heterogeneous sample (enzyme in turnover conditions). Determination of multiple structures from one grid. Local resolution analysis to understand dyamics. High resolution to analyze single amino acid side-chain movement.
Complimentary techniques: Protein design. Metal-mediated protein oligomerization to generate extended molecular assemblies. Analytical ultracentrifugation. Electron diffraction.
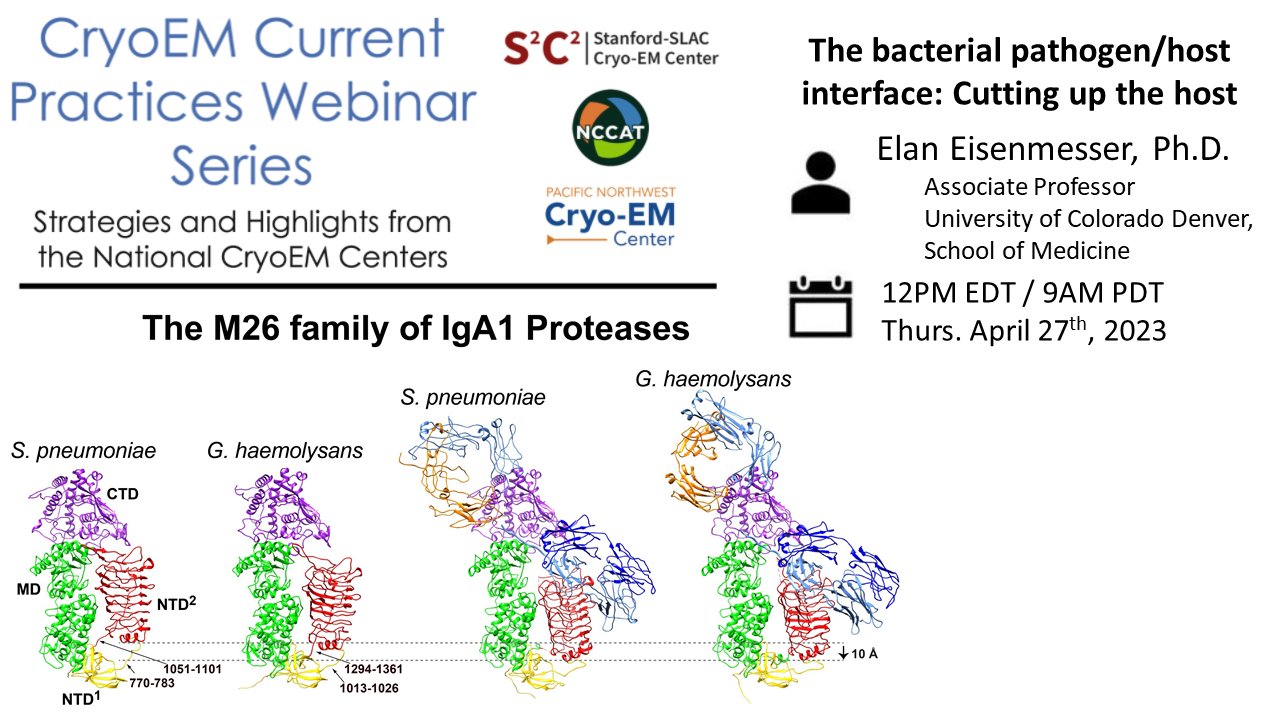
Tips & tricks of note:
Sample preparation: Using NMR analysis of point-mutants to choose an ideal cryoEM sample. Ideas for overcoming preferred orientation.
Data processing: Understanding substrate specificity by solving multiple structures.
Alternative methods: Site-directed mutagenesis, NMR as a complementary technique, RMSD analysis of substrate bound/unbound structures.
Tips & tricks of note:
Sample preparation: Blot-free vitrification to overcome preferred orientations
Data collection: Tilted data collection to overcome preferred orientations
Data processing: Using neural network particle pickers to find harder-to-pick views of non-spherical particles
Tips & tricks of note:
Sample preparation: Use of C. elegans for native source expression of a mechanosensing protein. Purification of samples from native sources (including tag choice). Fluorescence-detection size-exclusion chromatography (FSEC) to optimize buffer conditions. Continuous-carbon support to increase sample concentration for precious samples. Amylamine treatment of cryoEM grids to alleviate preferred orientation and improve ice thickness.
Data processing: Heterogeneous refinement to reconstruct multiple conformations from a single data set. Masked local refinement to improve resolution a weak binding partner. Observation of phospholipids in reconstrution.
Complementary techniques: Mass spectrometry to characterize sample composition. Molecular dynamics to probe role of membrane deformation in mechanism.
Tips & tricks of note:
Data processing: Practicalities of neural network picker software installation and usage. How to choose a particle picker & validation. Neural network picking for single particle analysis, filaments, & tomography. Interplay of particle picking and preferred orientations. Particle centering.

Tips & tricks of note:
Sample preparation: Synthetic G-quadruplex design. Screening of different grid types. Micrograph evaluation for a small (sub-30 kDa) particle.
Data processing: Elliptical blob picking. Heterogenenous refinement and 3D variability to separate conformational heterogeneity. Non-uniform refinement.
Complementary techniques: Analytical Ultracentrifugation. 1H-NMR. Small angle X-ray Scattering (SAXS) for size and flexibility estimation. Molecular dynamics with cryoEM map derived restraints. Molecular dynamics of a cryoEM derived model. Computational drugability analysis.

Tips & tricks of note:
Sample preparation: Transmembrane proteins, HEK293 freestyle cell expression, purification using FLAG-tag, pH-dependent enzyme activity, thermal denaturation, golgi apparatus, muscular dystrophy, IEX, SEC-MALS, SAXS
Data processing: 2-D classification, template-based picking, unbinned repicking to eliminate bias and improve resolution, preferred orientation.

Tips & tricks of note:
Sample preparation: Protein purification from red blood cells using glycerol gradients & gel filtration, detergent solubilization & purification, detergent screening, digitoxin solubilization, mass photometry.
Data collection: Single particle analysis & Cryo-ET
Data processing: Particle heterogeneity, non-uniform refinement, class-based processing. Tomogram 2-D slices, Sub-tomgram averaging.

Tips & tricks of note:
Sample preparation: Fibril extraction from heart tissue, mutation-specific perturbations, amyloid seeding, use of structure-based inhibitors.
Data processing: Manual picking, conformational heterogeneity, local resolution analysis, featureless cylinder, helical processing, twist and local maxima.
Complimentary techniques: X-ray microcrystallography.

Tips & tricks of note:
Sample preparation: Screening of: detergents, nano disc size, temperatures, grid type, and plunge freezer type. Use of pre-heated tools for temperature sensitive samples.





Tips & tricks of note:
Sample preparation: Protein prep and grid freezing optimization, glutaraldehyde cross-linking
Data collection: Tilted data collection to overcome preferred orientations
Data processing: Tips for processing tilted data, particle picking tips for tricky particles, helical filament processing
A copy of this presentation is available on the Open Science Framework (OSF) repository: https://osf.io/x7dv8/

Tips & tricks of note:
Data processing: computational infrastructure and resources
Tips & tricks of note:
Sample preparation: Optimization of grid freezing conditions
Data processing: Tips for dynamic membrane proteins
Tips & tricks of note:
Sample preparation: Testing detergents and nanodisks for integral membrane proteins.
Data collection: Optimizing pixel size and data collection rate.
Data processing: Using symmetry during 3D reconstruction; cleaning particle stacks with 3D classification.

Tips & tricks of note:
Sample preparation: Sample QC using differential scanning calorimetry & negative stain EM. The effects of temperature on protein quality and grid preparation.
