Vitrobot Standard Operating Procedure
1. Purpose
Setting up, operating, and shutting down the ThermoFisher Scientific (TFS) Vitrobot Mark IV in a safe and effective manner for the preparation of frozen cryo-EM of samples
2. Definitions:
2.1 ThermoFisher Scientific (TFS) Vitrobot Mark IV is a vitrification device for freezing protein samples for CryoEM analysis.
2.2 Liquid Nitrogen (LN2) is a cryogenic liquid stored under pressure.
2.3 Ethane is classified as a flammable gas.
3. Supplies & Equipment
- PPE (BSL-1)
- Laboratory Coat
- Nitrile Gloves
- Goggles / Safety Glasses
- Cryogenic Gloves
- Face Mask
- Chemicals/Reagents
- Liquid Nitrogen
- Ethane
- 70% Ethanol
- Distilled Water
- Sample
-
- Filter Paper (Pre-punched Whatman #1 or #541)
- Pipette and Tips
- Vitrobot Tweezers
- Glow Discharged / Plasma Cleaned EM Grids
- Grid Storage Boxes/Buttons
- Button Lid Tool (Screwdriver or TFS Pin Tool)
- LN2 Dewars for Transfer
- Forceps (fine tip and long tweezers)
- If BSL-2, place absorbent bench paper before starting work
4. Procedure
4.1 Instrument setup
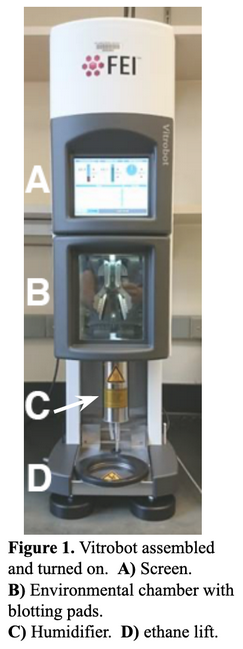
4.2 Cooling the Ethane
Safety note – Liquid ethane is a rapid cryogen and can burn and cause damage to your skin and eyes. You must wear proper PPE and take caution when dispensing. If the ethane surface freezes while cooling, it may build pressure and splash.



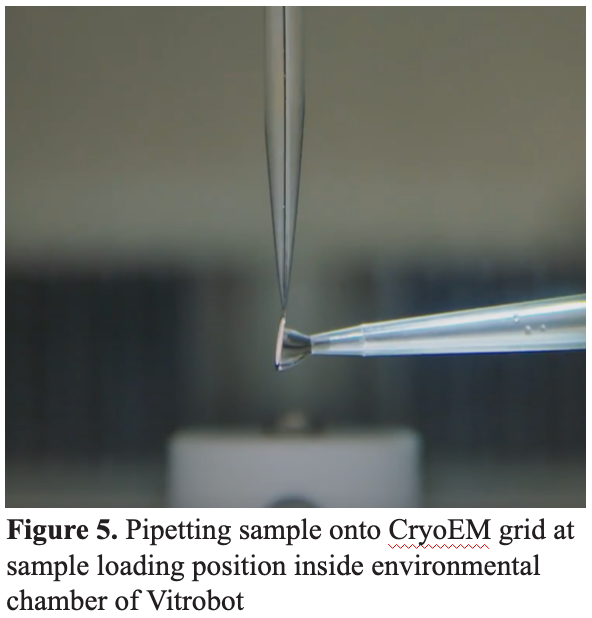
4.3 Freezing Cycle / Plunge Freezing
4.4 Revitalizing the Ethane
4.5 Shutdown
-
- Dry and store the Vitrobot tweezers.
- Tap the Exit button on the Vitrobot screen.
- Wait for the Vitribot to shut down: the screen will go completely blank and black
- Flip off the power switch on the back of the Vitrobot.
- Remove and dispose of blotting papers. Store rings inside chamber.
- Remove and empty the humidifier. Invert and dump humidifier at least 3 times to clear reservoir.
- Place the humidifier upside-down in a Styrofoam container for storage.
- Place the foam base containing liquid nitrogen and ethane container in the hood to evaporate.
- Ensure the ethane tank valves are closed.
5) Chemicals:
-
- Ethane
- Liquid Nitrogen
- Ethanol 70%
- Bleach (NaOCl)
6) Waste Disposal:
-
- Follow facility procedure for proper disposal.
- Biohazardous waste will be collected in designated bins lined with red biohazard bags.
- Chemical hazardous waste will be segregated by hazard class (e.g. flammable, corrosive) and state (e.g. solid, liquid), appropriately labelled, and placed in the laboratory’s hazardous waste cabinet.
- Additional Waste Guidelines [BSL-2]:
- Decontaminated solutions with 10% bleach (final conc.) will be disposed as chemical hazard liquid waste into the 10% bleach solution collection bottle.
- Bench paper used to protect surfaces is to be folded and discarded in the designated biohazard material bin.
